Bioorthogonal Reactions
Bioorthogonal chemistries to image and label proteins
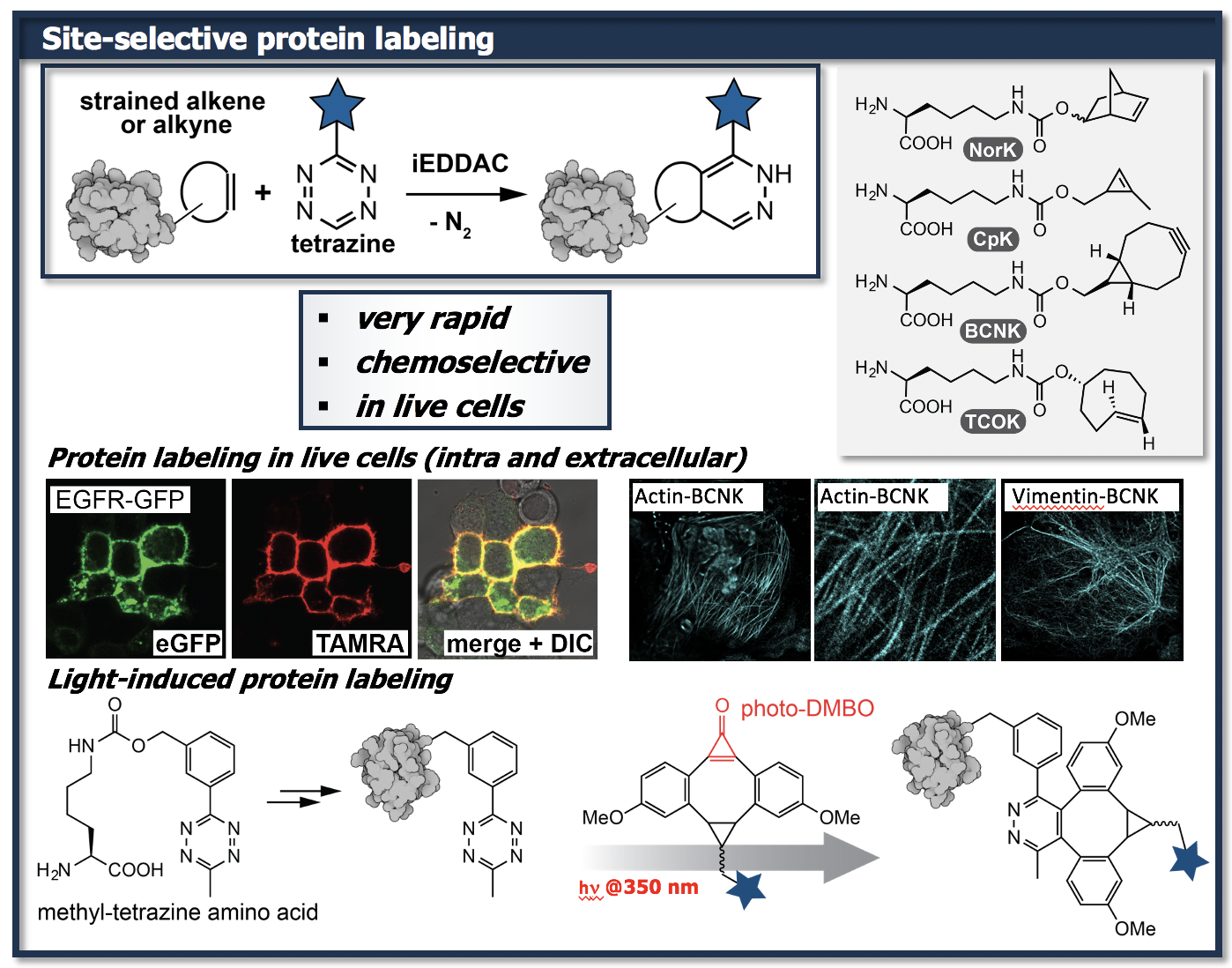
The site-specific incorporation of ncAAs with bioorthogonally reactive functional groups that subsequently allow rapid, chemoselective, site-specific labeling of the protein of interest (POI) with a custom-designed probe represents a technique with enormous potential.
This strategy provides a modular approach for installing diverse probes with a single genetic system, and also removes limitations that the translational machinery places on the size of probes.
We have developed various ncAAs that are able to engage in inverse electron-demand Diels-Alder cycloadditions (iEDDACs) and allow rapid and selective labeling of cell-surface and intracellular proteins in E. coli and mammalian cells. Furthermore, these approaches have found application for selectively inhibiting a specific target protein within living cells, via covalently tethering a promiscuous inhibitor to a specific enzyme.
We have recently developed a novel light-induced iEDDAC reactions between tetrazines and a cyclopropenone caged dibenzoannulated bicylo[6.1.0]nonyne probe (photo-DMBO). In-situ light activation of photo-DMBO conjugates allows rapid labeling of tetrazine-modified proteins in living E. coli. This new chemistry offers exciting opportunities to modify proteins in living cells in a spatio-temporally controlled manner.
Selected publications:
- S.V. Mayer, A. Murnauer, M.K. von Wrisberg, M.L. Jokisch, K. Lang*; Photo‐induced and Rapid Labeling of Tetrazine‐Bearing Proteins via Cyclopropenone‐Caged Bicyclononynes; Angew. Chem. Int. Ed. 2019, 58, 15876
- S. Mayer and K. Lang*, Tetrazines in Inverse-Electron-Demand Diels–Alder Cycloadditions and Their Use in Biology; Synthesis 2017, 49, 830.
- S.L. Scinto, D.A. Bilodeau, R. Hincapie, W. Lee, S.S. Nguyen, M. Xu, C.W. am Ende, MG Finn, K. Lang, Q. Lin, J.P. Pezacki, J.A. Prescher, M.S. Robillard, J.M. Fox*; Bioorthogonal chemistry; Nat Rev Methods Primers 2021, 1, 30
- K. Lang*, J.W. Chin*; Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins; Chem. Rev. 2014, 114, 4764
- K. Lang*, J.W. Chin*; Bioorthogonal reactions for labeling proteins; ACS Chem. Biol. 2014, 9, 16.