Crosslinking Reactions
Crosslinking chemistries to study protein-protein interactions
Protein-protein interactions are central to many biological processes. A considerable challenge consists however in understanding and deciphering when and how proteins interact with each other and with other biomolecules in their vicinity, and this can be particularly difficult when interactions are weak and transient.
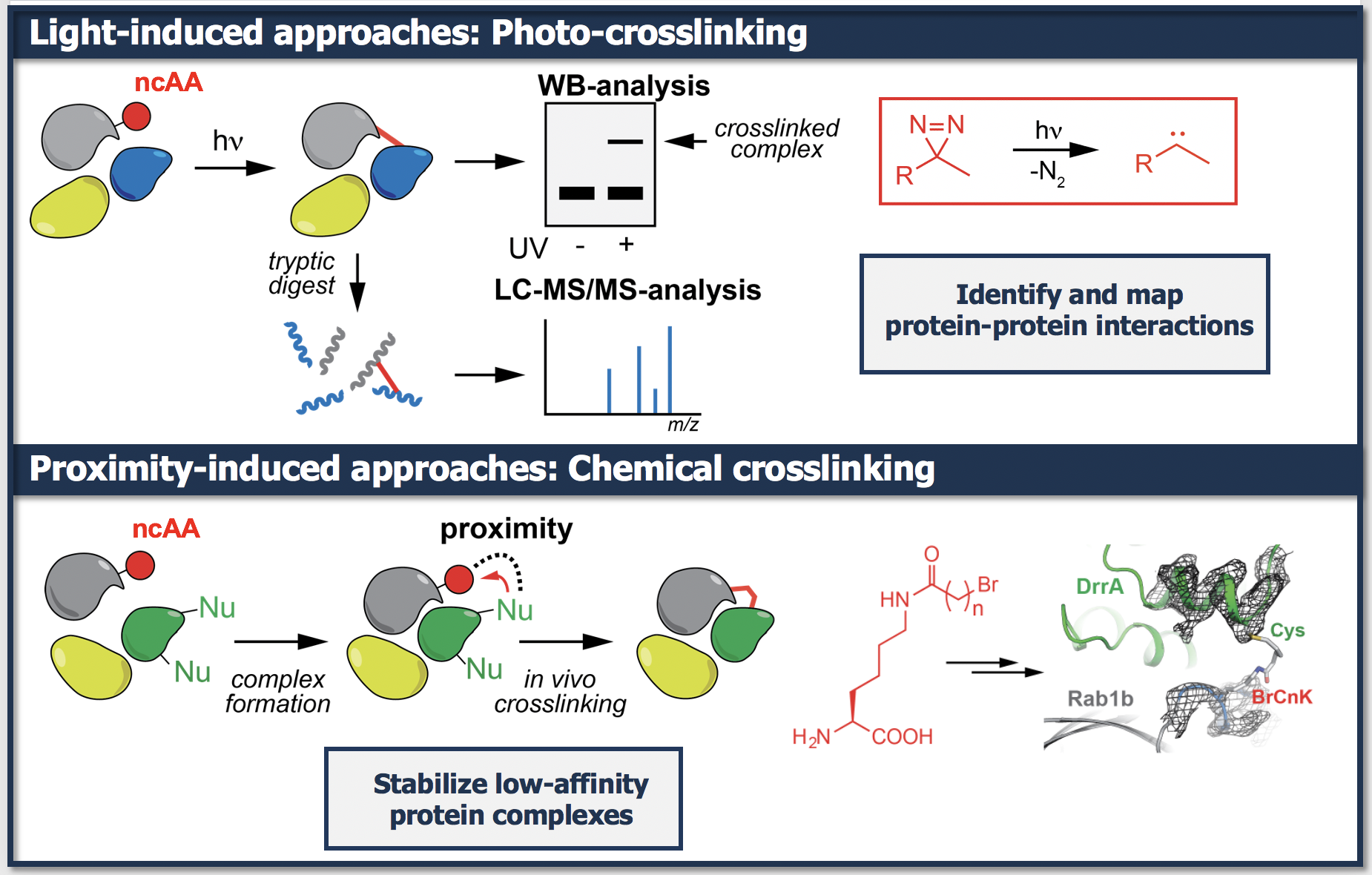
The site-specific incorporation of ncAAs bearing biologically inert functionalities such as benzophenones, aryl azides, and diazirines that upon UV-light irradiation form highly reactive intermediates and thereby crosslink with nearby molecules in response to light provides a powerful tool for mapping transient protein-protein interactions (photocrosslinking) and identifying novel protein interactors.
Complimentary strategies consist in site-specific incorporation of ncAAs bearing finely tuned electrophilic moieties (e.g bromoalkyl moieties) that react with natural nucleophilic amino acids in a proximity-dependent manner upon protein complex formation. We have pioneered the use of proximity-triggered crosslinking approaches to covalently stabilize low-affinity and transient protein complexes in living bacteria and mammalian cells to further their structural elucidation.
Selected publications:
- T.A. Nguyen, T.F. Gronauer, T. Nast‐Kolb, S.A. Sieber*, K. Lang*; Substrate Profiling of Mitochondrial Caseinolytic Protease P via a Site‐Specific Photocrosslinking; Approach; Angew. Chem. Int. Ed. 2022, e202111085
- J. Du, M.K. von Wrisberg, B. Gulen, M. Stahl, C. Pett, C. Hedberg, K. Lang*, S. Schneider*, A. Itzen*; Rab1-AMPylation by Legionella DrrA is allosterically activated by Rab1; Nat. Commun. 2021, 460, 12
- T.A. Nguyen, M. Cigler, K. Lang*; Expanding the Genetic Code to Study Protein–Protein Interactions; Angew. Chem. Int. Ed. 2018, 57, 14350
- M. Cigler, T.G. Müller, D. Horn-Ghetko, M.K. von Wrisberg, M. Fottner, R. S. Goody, A. Itzen, M.P. Müller and K. Lang*; Proximity-triggered covalent stabilization of low affinity protein complexes in vitro and in vivo; Angew. Chem. Int. Ed. 2017, 56, 15737.